Sajid Hussain

TOPIC / ACTIVITIES:
ADVANCED OXIDATION PROCESSES
Advanced oxidation processes (AOPs) have recently received a great interest in the research world. A lot of processes can be numbered in this kind of treatments, not many experimental are available for industrial applications. In particular AOPs on priority and emerging pollutants in water wastewater and liquid waste should be deepen in knowledge to introduce them in industrial exploitations.
The PhD programm aims to study some AOPs and their application in a regional industrial tissue.
PhD Project
The aim of this project is to devise new Fenton like AOPs for the treatment of liquid wastes. First, a number of Iron, Copper, and Silver based catalysts supported over Zirconia and co-catalyzed with Strontium have been used for the treatment of Ibuprofen contaminated aqueous solutions at standard conditions; 70oC of temperature, catalyst dose of 250 mg/L, oxidant dose (3%) of 25 ml/L, pH 5, pollutant dose of 10 mg/L and reaction time of 2 hours. On the basis of Ibuprofen degradation, TOC abatement, and projected toxicity of treated samples, three catalysts namely; Zr5Cu, Zr5Fe, and Zr10Sr5Cu have been marked as highly active. In order to enhance the TOC abatement efficiency of these catalysts, the operating conditions have been varied; temperature from 50 to 90oC, catalyst dose from 250 to 600 mg/L, oxidant dose from 12.5 to 30 ml/L, pH from 3 to 10, pollutant dose from 10 to 50 mg/L, and reaction time from 30 min to 180 min (In Progress).
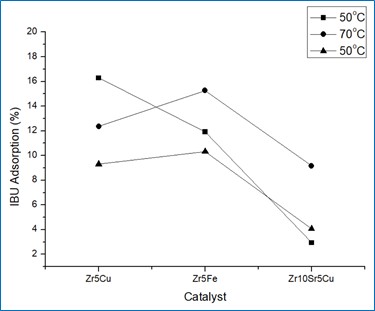
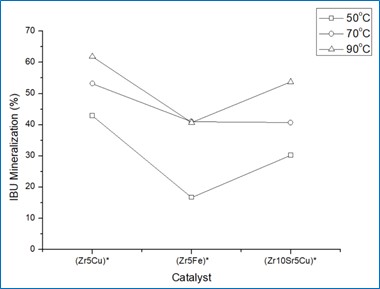
PROGRESS OF THE ACTIVITIES
Effect of temperature on Ibuprofen adsorption over catalyst surface and effect of temperature on catalytic abetment of Ibuprofen.
Pubblications
S. Hussain, E. Aneggi, S. Briguglio, M. Mattiussi, V. Gelao, I. Cabras, L. Zorzenon, A. Trovarelli, D. Goi, “Enhanced ibuprofen removal by heterogeneous-Fenton process over Cu/ZrO2 and Fe/ZrO2 catalysts” JOURNAL OF ENVIRONMENTAL CHEMICAL ENGINEERING, Volume 8, Issue 1, February 2020, 103586.
Hussain, S; Aneggi, E; Goi, D; Trovarelli, A; “Bimetallic Cu/Fe Catalysts for Ibuprofen Mineralization”, 2021, CATALYSTS, 11 (11), DOI10.3390/catal11111383.
Hussain, S; Aneggi, E; Trovarelli, A; Goi, D; “Heterogeneous Fenton-like oxidation of ibuprofen over zirconia-supported iron and copper catalysts: effect of process variables”, 2021, JOURNAL OF WATER PROCESS ENGINEERING, 44, DOI10.1016/j.jwpe.2021.102343.
Hussain, S; Aneggi, E; Maschio, S; Contin, M; Goi, D; “Steel Scale Waste as a Heterogeneous Fenton-like Catalyst for the Treatment of Landfill Leachate”, 2021, INDUSTRIAL & ENGINEERING CHEMISTRY RESEARCH, 60 (31):11715-11724, DOI10.1021/acs.iecr.1c01901.
Hussain, S; Aneggi, E; Trovarelli, A; Goi, D; “Heterogeneous Fenton-like oxidation of ibuprofen over zirconia-supported iron and copper catalysts: effect of process variables”, 2021, JOURNAL OF WATER PROCESS ENGINEERING, 44, DOI10.1016/j.jwpe.2021.102343.
Hussain S., Aneggi E., Goi D. (2021). “Catalytic activity of metals in heterogeneous Fenton‑like oxidation of wastewater contaminants: a review”, ENVIRONMENTAL CHEMISTRY LETTERS, https://doi.org/10.1007/s10311-021-01185-z.
Hussain, S; Aneggi, E; Trovarelli, A; Goi, D; "Removal of Organics from Landfill Leachate by Heterogeneous Fenton-like Oxidation over Copper-Based Catalyst”, 2022, CATALYSTS,12 (3), DOI10.3390/catal12030338.
Hussain, S; Aneggi, E; Comuzzi, C; Baderna, D; Zuccaccia, D; Trovarelli, A; Goi, D; "Abatement of the ecotoxicological risk of landfill leachate by heterogeneous Fenton-like oxidation”, 2023, ENVIRONMENTAL SCIENCE AND POLLUTION RESEARCH, 30 (8): 21025-21032, DOI10.1007/s11356-022-23682-6.
Aneggi, E; Hussain, S; Baratta, W; Zuccaccia, D; Goi, D; “Enhanced Heterogeneous Fenton Degradation of Organic Dyes by Bimetallic Zirconia-Based Catalysts”, 2024, MOLECULES, 29 (9), DOI10.3390/molecules29092074.
Hussain, S; Aneggi, E; Gelao, V; Briguglio, S; Mattiussi, M; Baratta, W; Zuccaccia, D; Trovarelli, A; Goi, D; "Potential Residual Toxicity of the Ibuprofen Oxidative Degradation Products by HPLC-MS and Principal Component Analysis”, 2024, ACS ES&T WATER, 4 (5): 2057-2063, DOI10.1021/acsestwater.3c00630.
